What is the PEACE-COS study?
PEACE-COS is an abbreviation of Person-centred outcome measures in End-of-life cAre in Critical carE Core Outcome Set. It is a research study that aims to develop consensus opinion and use family and expert experience to inform the development of a person-centred core outcome set for end-of-life care in critical care.
Why is it important?
At the moment, researchers use a range of different outcome measures to evaluate end-of-life care in critical care. For example, one research study may measure how long patients stayed in critical care while another study may measure what treatment patients received. Using different outcome measures makes it difficult to compare or combine study results and increases the likelihood of outcome reporting bias, which occurs when several outcomes are measured but only significant findings are reported. This means that some research findings are not used to improve current and future care.
Few studies look at outcomes from the patient or family perspective. We don’t understand which outcomes matter most to the person at the centre of the care (the patient) or their family. Person-centred care includes both the patient and their family.
Research in other health areas has shown that patients and their families can have very different opinions as to which outcomes matter most when compared to health professionals looking after them. The PEACE-COS study is specifically designed to ensure that family and expert opinions are all reflected in the core outcome set developed.
What is a Core Outcome Set (COS)?
A Core Outcome Set (COS) brings together an agreed list of outcomes that all research studies should measure and report as a minimum in a specific health area. It is made up of two parts – ‘what’ to measure and ‘how’ to measure. The PEACE-COS study is part of a wider piece of research and will focus on ‘what’ should be measured.
How will the PEACE-COS study be undertaken?
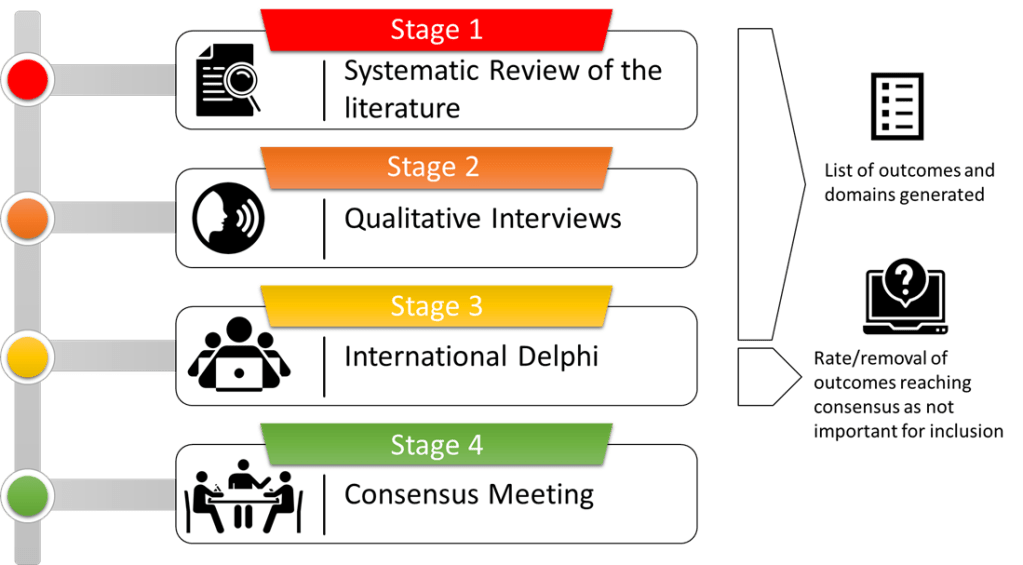
The PEACE-COS study involves four interlinked stages:
Stage 1: A systematic review of the literature to identify outcomes and measurement tools reported in research studies.
Stage 2: An interview study with family members that have experienced end-of-life care in critical care to find out their views on what they identify as important outcomes. An interview/focus group study with healthcare professionals who have professional experience of end-of-life care in critical care to find out their views on what they identify as important outcomes.
Stage 3: A modified international e-Delphi study where healthcare professionals, researchers and family members that have experienced end-of-life care in critical care will be invited to rate the importance of the outcomes identified in stages one and two and suggest other potential measures that may have been missed in a series of rounds.
Stage 4: A consensus group meeting and initial formulation of draft COS of ‘what’ should be measured.